Background:
Invasive mold infections (IMI) are a leading cause of mortality in immunocompromised hosts. Children diagnosed with hematologic malignancies experience profound, prolonged neutropenia following intensive chemotherapy leading to augmented risk for infection-related outcomes, a risk that is mitigated with antimicrobial prophylaxis. Prior to the current study, we conducted a single-institution retrospective review of children diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), or lymphoma between 2006-2015 and determined the incidence of IMI at our institution to be 4.8% (47/976), with an exceptionally high incidence observed in patients with AML (8.1%, 9/111). This observation prompted a change in clinical practice to broaden mold coverage in high risk populations, including development of a risk-stratified algorithm for antifungal prophylaxis. For example, fluconazole was replaced with micafungin (inpatient) or voriconazole/posaconazole (outpatient) for newly diagnosed AML and relapsed/refractory ALL/AML, but still employed for populations at lower risk for IMI (infant ALL, Down syndrome ALL, ALL with steroid-induced diabetes). The objective of the present study was to evaluate the change in IMI incidence post-implementation of this algorithm, and to identify host factors contributing to risk for IMI in children with hematologic malignancies.
Methods:
We conducted a second retrospective review of children ≤ 21 years old and diagnosed with ALL, AML, or lymphoma during the follow-up period from 2016-2019, and diagnosed with IMI between 2016 and June 2020. To identify potential cases, we employed a strategy identical to the one used in the 2006-2015 review, specifically, a search of the electronic medical record utilizing ICD9 codes broadly inclusive of relevant cancer and fungal diagnoses. Each potentially eligible case was then reviewed for the following inclusion/exclusion criteria: diagnosis and treatment of ALL, AML, or lymphoma at Texas Children's Hospital, diagnosis of IMI that met criteria for 'proven' or 'probable' per the European Organization for Research and Treatment of Cancer/Mycoses Study Group and occurring prior to stem cell transplant, and no underlying immunodeficiency or history of solid organ transplant. Host and disease-related factors, as well as IMI incidence, were compared for 2006-2015 vs. 2016-2020 using a Chi-square, Fisher, or Student t-test as appropriate, and host factors predictive of IMI were assessed by multivariable linear regression.
Results:
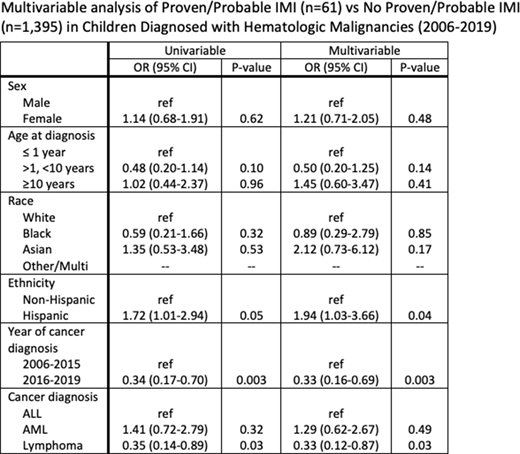
The overall incidence of proven/probable IMI in children diagnosed with hematological malignancies between 2006-2019 was 4.2% (61/1456). The incidence of IMI decreased from 4.8% to 2.9% between 2006-2015 and 2016-2020. For specific diagnoses, the rate of IMI decreased from 5.0% to 3.6% (ALL, 35/705 vs. 10/276), from 1.9% to 1.4% (lymphoma, 47/976 vs. 14/480), and from 8.1% to 3.2% (AML, 9/111 vs. 2/62). No significant differences in host factor or disease-related characteristics were noted when comparing IMI cases in 2006-2015 vs. 2016-2020, nor were there differences in the proportion of patients in relapse at the time of IMI or taking antifungal prophylaxis. Substantial differences in representative mold species were noted between the two time periods, e.g. Aspergillus spp. accounted for 19/47 IMI from 2006-2015, but accounted for none of the IMIs diagnosed 2016-2020. In 2016-2020, 5/14 IMI were due to Trichosporon spp., with 4/14 Rhizopus spp., 2/14 Fusarium spp., 1/14 Curvularia spp., 1/14 Histoplasma spp., and 1 that met criteria for probable IMI. In multivariable analyses (Table 1), Hispanics were more likely to develop IMI than non-Hispanics (p=0.04, OR 1.94, CI 1.03-3.66), and those with lymphoma were less likely to develop an IMI than those with ALL (p=0.03, OR 0.33, CI 0.12-0.87). Patients diagnosed between 2016-2019 were substantially less likely to develop IMI than those diagnosed 2006-2015 (p=0.003, OR 0.33, CI 0.16-0.69).
Conclusion:
In this single-institution study, risk for IMI in children with hematologic malignancies declined significantly after implementation of an antifungal prophylaxis algorithm that broadened coverage for high risk populations. Hispanics were at higher risk for IMI than non-Hispanics, suggesting a need to investigate relevant factors contributing to this disparity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.